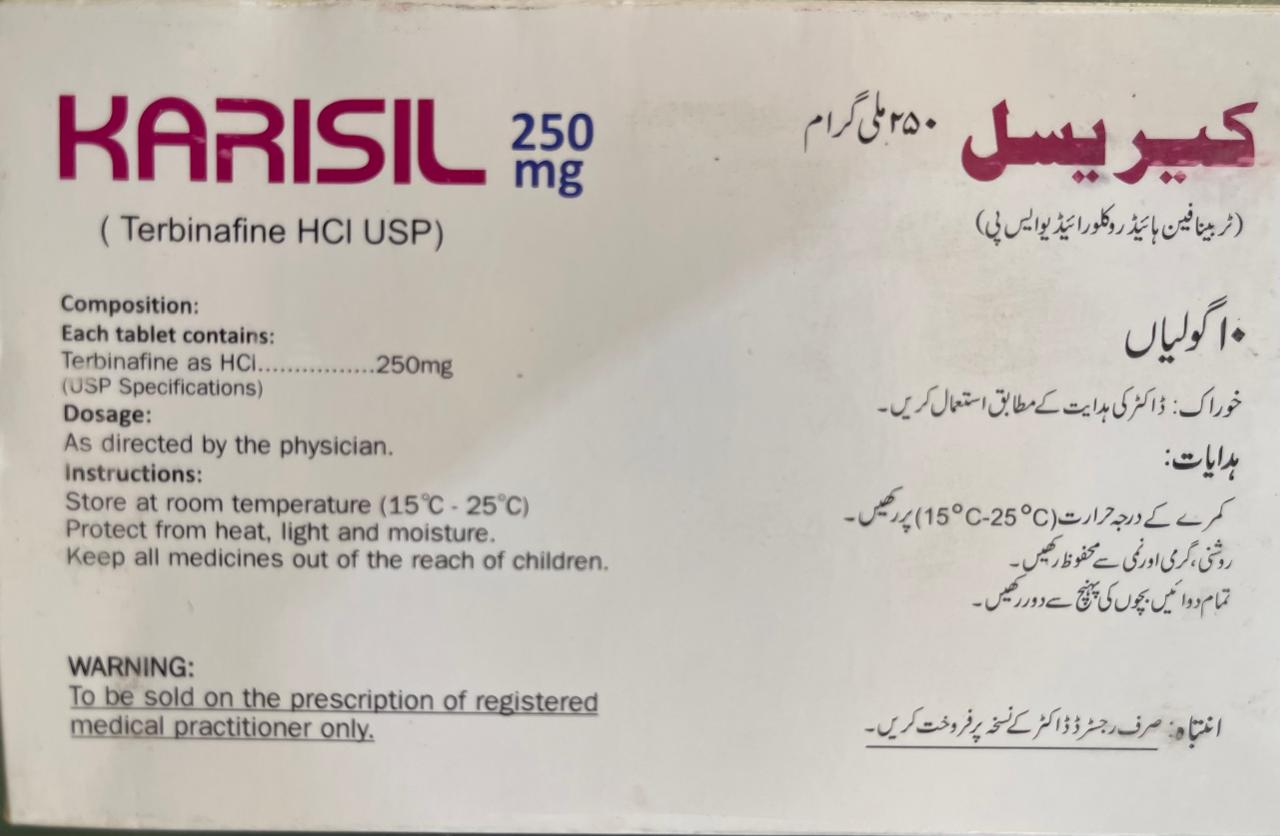
**KARISIL 250MG TAB (Terbinafine) Description**
**Drug Class**:
– Allylamine antifungal agent.
**Mechanism of Action**:
– Inhibits **squalene epoxidase**, an enzyme required for ergosterol synthesis (essential for fungal cell membranes). Accumulation of squalene causes fungal cell death.
**Indications**:
– **Dermatophyte infections**: Onychomycosis (fungal nail infections), tinea corporis (ringworm), tinea cruris (jock itch), tinea pedis (athlete’s foot).
– Occasionally used off-label for cutaneous candidiasis or pityriasis versicolor.
**Pharmacokinetics**:
– **Absorption**: Bioavailability ~40% (improved with fatty meals).
– **Distribution**: Concentrates in skin, nails, and adipose tissue (long-lasting effects).
– **Metabolism**: Hepatic (CYP2C9, CYP3A4, and others).
– **Excretion**: Renal (70–80%); half-life ~200–400 hours (due to tissue accumulation).
**Dosage & Administration**:
– **Adults**: 250 mg once daily.
– **Duration**:
– **Skin infections**: 2–6 weeks.
– **Fingernail infections**: 6 weeks.
– **Toenail infections**: 12 weeks.
– **Note**: Continue treatment until clinical resolution; therapeutic effects may persist post-treatment due to tissue retention.
**Contraindications**:
– Hypersensitivity to terbinafine or allylamines.
– Chronic or active liver disease.
– Severe renal impairment (CrCl ≤50 mL/min: avoid or adjust dose).
**Drug Interactions**:
– **CYP2D6 inhibitors/substrates**: SSRIs (e.g., paroxetine), beta-blockers (e.g., metoprolol), TCAs (e.g., amitriptyline) – may increase their levels.
– **Rifampin**: Reduces terbinafine levels (enzyme induction).
– **Caffeine**: Metabolism may be inhibited (increased caffeine effects).
**Side Effects**:
– **Common**: Nausea, diarrhea, abdominal pain, headache, taste disturbances (dysgeusia), rash.
– **Serious**: Hepatotoxicity (monitor LFTs), blood dyscrasias (e.g., neutropenia), Stevens-Johnson syndrome.
**Warnings & Precautions**:
– **Hepatotoxicity**: Monitor liver function (baseline and during treatment).
– **Pregnancy**: Category B (use cautiously; limited data).
– **Lactation**: Avoid (excreted in breast milk).
– **Taste loss**: Reversible upon discontinuation.
**Patient Counseling**:
– Take with food to enhance absorption.
– Complete the full course, even if symptoms improve.
– Report signs of liver injury (jaundice, dark urine, fatigue) or allergic reactions.
– Avoid alcohol (risk of liver stress).
**Storage**:
– Store below 25°C, protected from light and moisture.
This summary highlights key aspects of Karisil (terbinafine). Always verify dosing and safety based on patient-specific factors (e.g., liver/kidney function, comorbidities).













Reviews
There are no reviews yet.